Describe the Electron Density Around Electrophiles
However due to the uncertainty principle its not possible to identify the exact location of an electron at any instant in time. Weak acids are good leaving groups.
Nucleophiles And Electrophiles Organic Chemistry Tutor
Most electrophiles are positively charged have an atom that carries a partial positive charge or have an atom that does not have an octet of electrons.

. In benzene π electrons are delocalisedspread out 1In alkenes π electrons are concentrated between 2 carbons 1Electrophiles attracted more to greater electron density in alkenes 1 Describe with the aid of suitable diagrams the bonding and structure of a benzenemolecule. Using our example in diagram 1 where is this. In chemistry an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair.
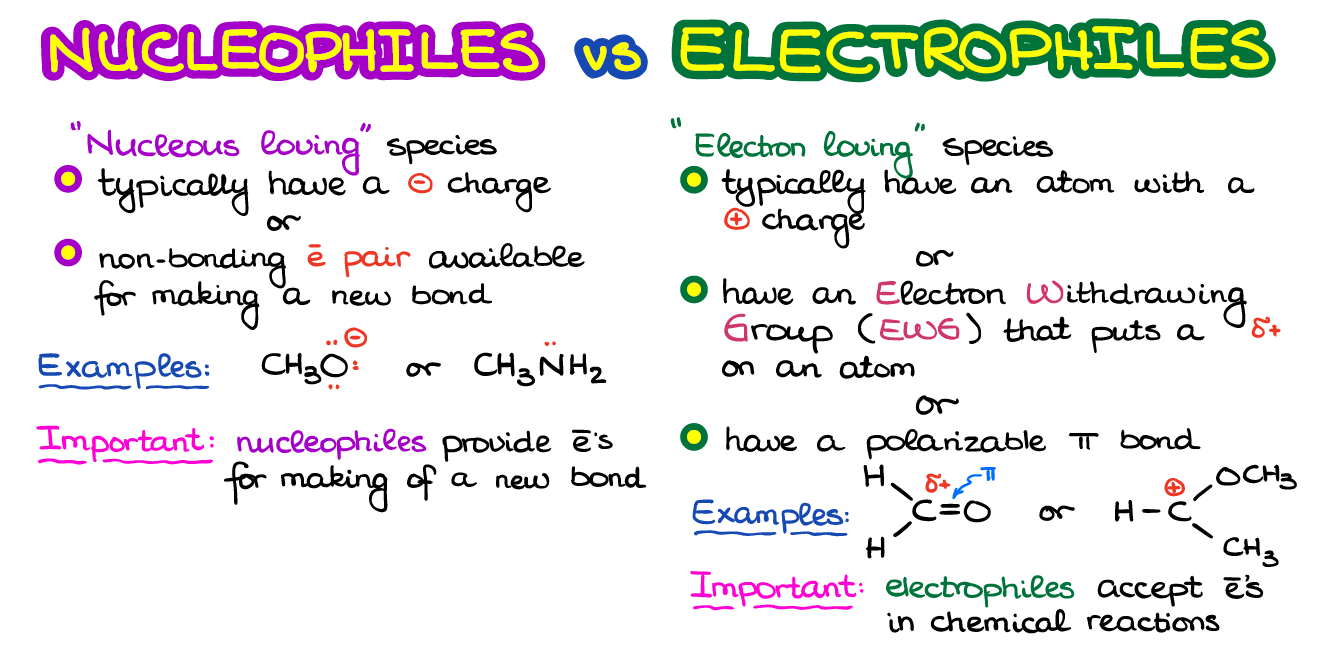
In simple terms it means electrons-loving. C-C sigma bond C-C p bond Electrophiles tend to be molecules where part of the molecules has a slight positive charge. The term electrophile can be split into electro derived from electron and phile which means loving.
Electrophiles will often have electron-withdrawing groups a group containing electronegative elements pulling the electron density towards themselves. These species are called electrophiles. Recall also that this trend can be explained by considering the increasing size of the electron cloud around the larger ions.
1 not relevant for electrophiles 2 relatively low 3 relatively high 4 unaffected 15Which description about leaving groups is correct 1. Whereas we speak of acidity to describe the strength of acids we speak of electrophilicity to describe the strength of electrophiles. For example in the picture at the beginning of this post we have a couple of electrophilic molecules.
In general the electron is more likely to be found in regions with high electron density. - They readily accept electrons to. Movement of electrons depends on the density.
Electrophiles mainly interact with. They readily accept electrons to form new bonds 7. Alternatively electrophiles may also have polarizable π-bonds such as CO or CN.
Favour electrophilic addition and electrophilic substitution reactions. They are electron deficient and hence electrons loving. Also called Lewis acid because they accept electrons.
Greater pi electron density around the ring the benzene ring in phenol is activated attracts electrophilesNO2 moremakes it more susceptible to electrophiles Diphenylethanedione is a pale yellow colour which disappears when it is reduced. They are positively charged or neutrally charged. Hydrogen ion in acids and methyl-carbocation are examples of electrophilic substances.
Describes the less common mode involving single electron reconfiguration. They attack electron rich parts of molecules. Nucleophiles high electron density always.
Recall that electrophiles attack the electron rich site of a molecule called a nucleophile. The colour results from the arrangement of the delocalised pi-electrons. It is a reagent that is characterized with a low density of electrons in its valance shell and therefore reacts with a high-density molecule ion or atom to form a covalent bond.
3 votes smithtanya20 5 years ago 312. This site is known to be a negatively charged region. The electron density around electrophiles is.
Strong acids are good leaving groups. An electrophilic addition reaction is an addition reaction which happens because what we think of as the important molecule is attacked by an electrophile. Because electrophiles accept electrons they are Lewis acids.
The movement of electron pairs is represented by curved arrows. Movement of electrons is affected by the density and the movement is generally from a high-density area to low-density area. Electrophiles are involved in electrophilic substitution and addition reaction.
Electrophiles E 1 to E 4 with LUMO energies as shown. - Relatively low - Electrophiles are area in a molecule that have a relatively low electron density. They are electron deficient.
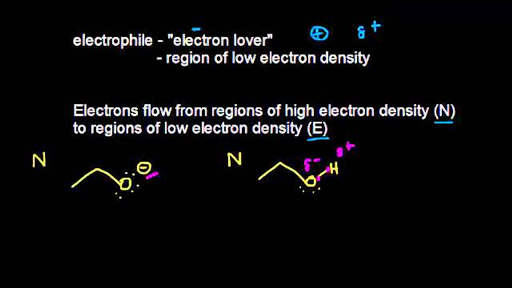
Organic chemists like to think of the movement of electrons as a flow of electron density from regions of high to regions of low electron density that is from nucleophiles to electrophiles. The chlorine-adjacent carbon in. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases.
Theres a good analogy between electrophiles and acids. Just as some acids are stronger than others some electrophiles are stronger than others. An arrow 2 electrons shared.
In chemical reactions what role do electrophiles play. What you need to know. In chemical reactions what role do electrophiles play.
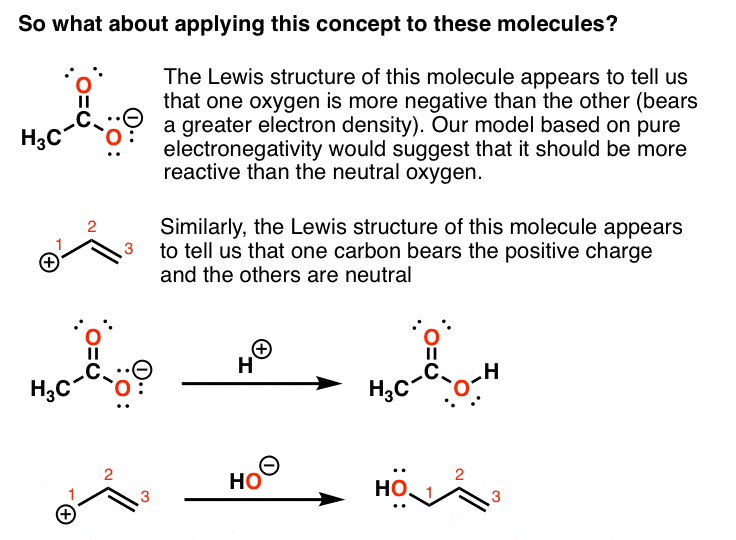
Electron density is a representation of the probability of finding an electron in a specific location around an atom or molecule. Ethanol is a nucleophile N because the O atom has lone pairs and a δ charge. An electron behaves as though it is spread out around the nucleus as a sort of cloud sometimes referred as an electron cloud.
It pulls electron density from the C atom so it becomes δ and the C atom becomes δ. They are therefore vulnerable to attack by species which are attracted to high electron density. In 1-chlorobutane the electronegative electron withdrawing chlorine atom draws electron density away from the adjacent carbon making it electron deficient.
By measuring the electron density around the nucleus it is possible to define regions where electrons are most likely to. Describe the electron density around electrophiles relatively low 6. They could be called electron deficient.
Strong bases are good leaving groups. Weak bases are good leaving groups. A nucleophile when the bond being made is a covalent bond in.
Curved arrows are used to indicate which way electrons are moving similar to how we show the movement of cations and anions in resonance structures. The important molecule has a region of high electron density which is attacked by something carrying some degree of positive charge. A Brønsted-Lowry base when the bond being made is to a proton.
A Lewis base when the bond being made is a dative or coordinate bond in other words relatively weak so that it repeatedly forms and dissociates at or near room temperature. Quick summary An electron-rich molecule is called. The electron density inherent in the negative charge is spread around a larger.
Ethyl chloride is an electrophile E because the δ C atom attracts the negative charge in other molecules. They move from high-density area to low density area.
Intro To Resonance In Organic Chemistry Master Organic Chemistry
Solved 1 Draw Resonance Structures And Identify Which Chegg Com

B3lyp 6 31g D Map Of The Total Electron Density Of The Transition Download Scientific Diagram

B3lyp 6 31g D Map Of The Total Electron Density Of The Transition Download Scientific Diagram

Solved Describe The Electron Density Around An Electrophile Relatively High Electron Density Is Not Relevant For Electrophlles Crelatively Low Unaffected
Identifying Nucleophilic And Electrophilic Centers Video Khan Academy
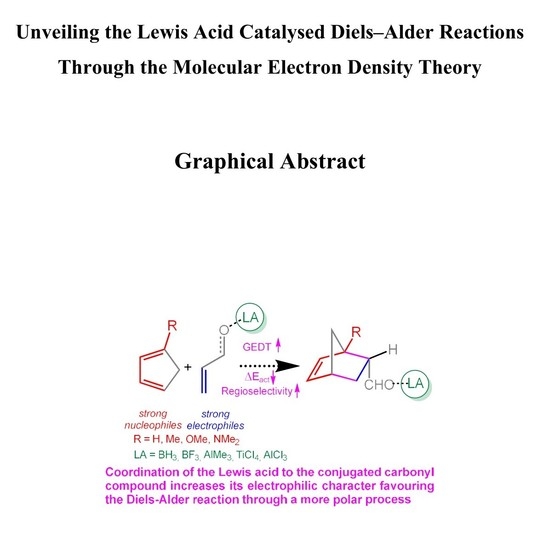
Molecules Free Full Text Unveiling The Lewis Acid Catalyzed Diels Alder Reactions Through The Molecular Electron Density Theory
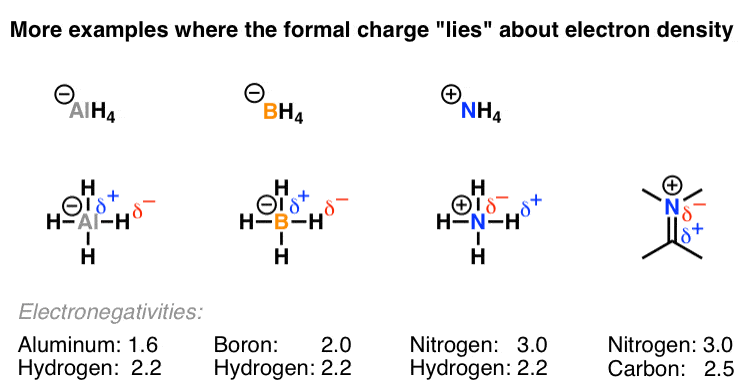
Common Mistakes In Organic Chemistry Formal Charges Can Mislead

Electrostatic Potential Mapped On The Surface Of Molecular Electron Download Scientific Diagram

Solved 14 The Electron Density Around Electrophiles Is 1 Not Relevant For Electrophiles 2 Relatively Low 3 Relatively High 4 Unaffected 15 Which Description About Leaving Groups Is Correct 1 Strong Bases Are Good
Solved What Does Electron Density In A Tiny Volume Of Space Mean

18 02 Identifying The Nucleophile Leaving Group And Electrophile Youtube

B3lyp 6 31g D Map Of The Total Electron Density Of The Transition Download Scientific Diagram
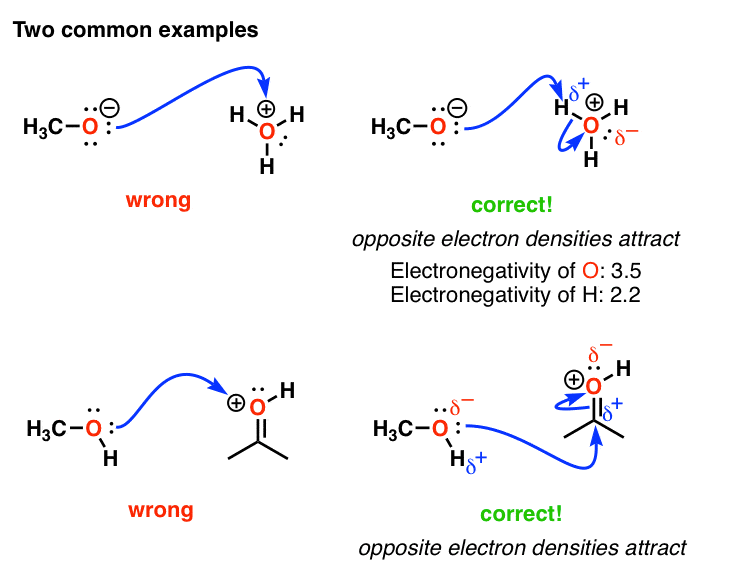
Common Mistakes In Organic Chemistry Formal Charges Can Mislead
General Organic Chemistry Finding Maximum Electron Density Physics Forums

Solved Describe The Electron Density Around An Electrophile Relatively High Electron Density Is Not Relevant For Electrophlles Crelatively Low Unaffected
General Organic Chemistry Finding Maximum Electron Density Physics Forums
What Makes A Good Nucleophile Master Organic Chemistry

Mpwb1k 6 31g D Maps Of The Total Electron Density And Elf Valence Download Scientific Diagram
Comments
Post a Comment